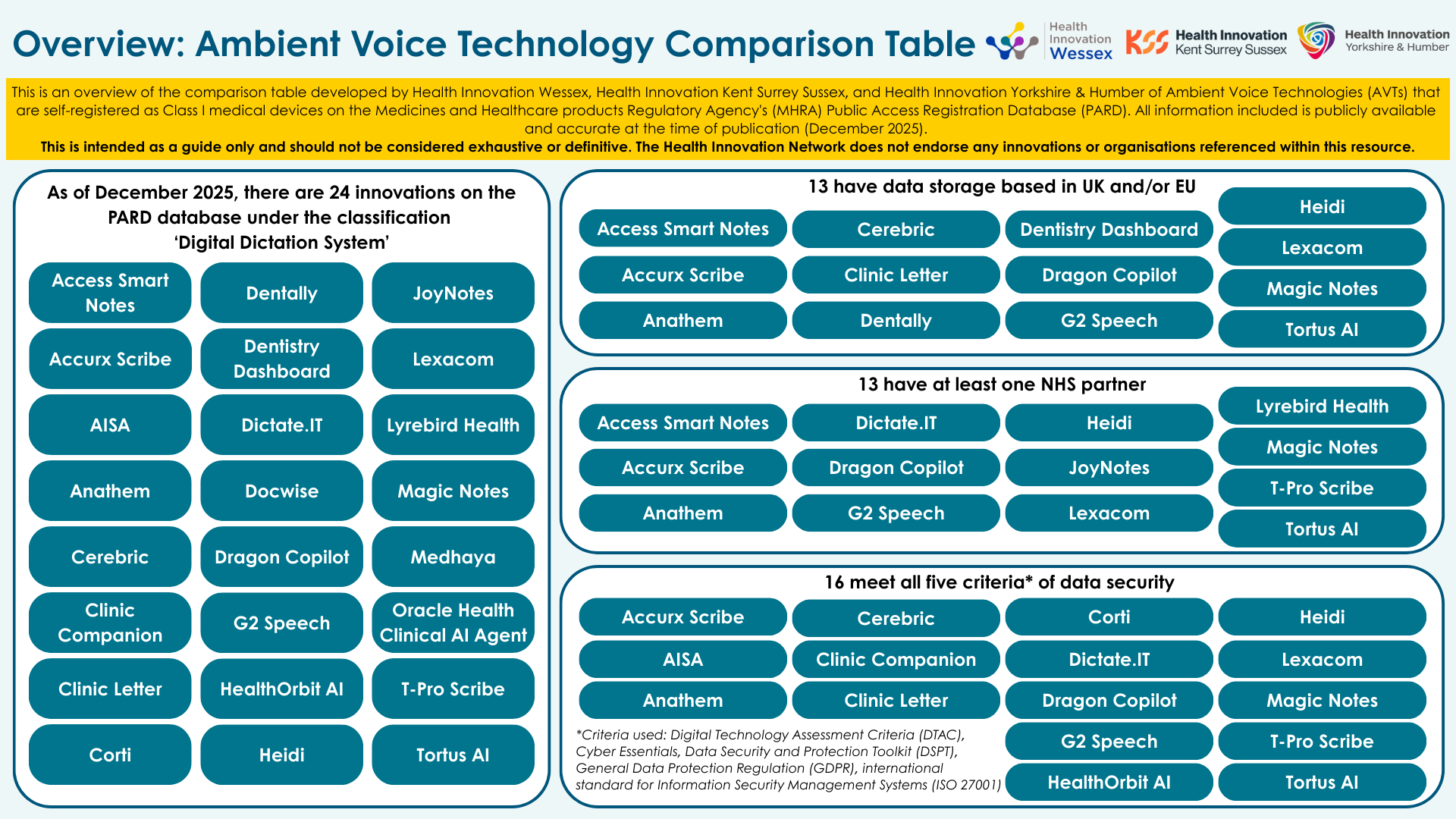
The Medicines and Healthcare products Regulatory Agency (MHRA) note 24 technologies that have Class I medical device status on the Public Access Registration Database (PARD). Six months ago, that number was eight.
Due to the fluid growth of the market, the number of technologies in this space is growing. With growth comes great opportunity and more options to choose from, making the market competitive.
However, it also brings added complication. More options mean that organisations need to review the technologies and understand what would be best to fit their needs. This takes time to review, compare, and understand the functionality of the technology on the market.
The most important thing is to know what you want to get out of the technology before you start looking for it. The benefits of freeing clinician time and improved note accuracy may be the ‘why’, but the ‘when’ and ‘how’ are just as crucial to ensure that you can safely use the technology to its full capability, while safeguarding patient confidence that technology enhances their care.
Health Innovation Wessex can support you in doing this using an evidence-based adoption process. We can undertake market reviews, share learning and best practice, support implementation of technology in this space and support how your organisation could best evaluate the change to understand the impact.
Ambient Voice Technology Comparison Table
To assist health and social care organisations in their search for the right AVT, Health Innovation Wessex, Health Innovation Kent Surrey Sussex, and Health Innovation Yorkshire and Humber have created an AVT comparison table that gives some basic information about all AVTs that are currently on the MHRA PARD and are all (self) certified Class I medical devices.
Please note that this document is intended for guidance only. The Health Innovation Network does not endorse any of the commercial products included within this document.
A visual overview is available below. The comparison table can be downloaded here or under the 'Useful Links' tab at the bottom of this page.